Quality
Quality by Design
Quality is a top priority at GCM. We are committed to delivering consistent product quality through manufacturing and service excellence that meets, and often exceeds, your expectations. We accomplish this through a structured process of product control plans, trained personnel, documentation and best-in-class metrology equipment.
We have the ability to provide critical design feedback on your product, create prototypes and test them to the stringent requirements of their industries. We have established procedures to provide PPAP, FMEA and Process Validations where required.
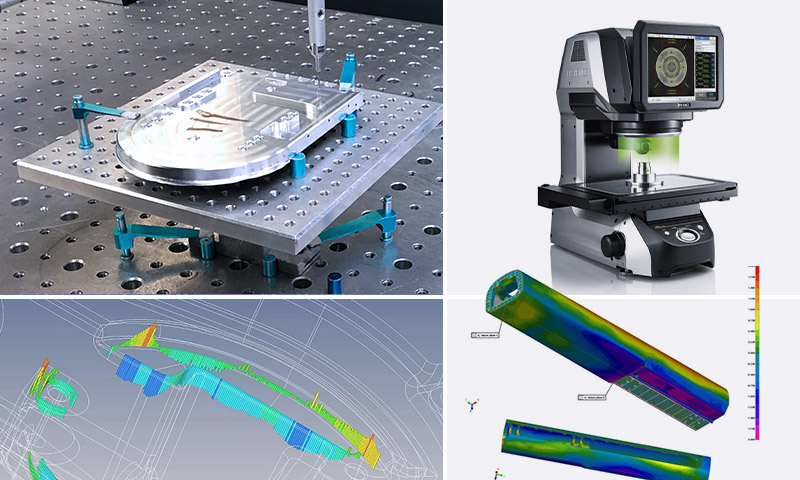
GCM invests in highly capable production and inspection equipment that is validated on installation to ensure superior performance. Our inspection equipment is statistically validated with measurement system analysis tools.
Certifications
GCM’s quality management systems are ISO and AS certified for Medical, and Aerospace standards. The standards for these certifications are embedded into our business operations and procedures which drive the continuous improvement needed for an effective quality management system.
- AS 9100D
- ISO 9001:2015
- ISO 13485:2016
- ISO 14001:2015
- NC AS 9100D / ISO 9001:2015
- NC ISO 13485:2016

Validation
GCM performs product and process validations for medical and aerospace applications. It is paramount that GCM provide our customer with precise components that repeatedly meet customer specifications and inspection criteria.
- PPAP
- Gauge R&R
- Process Validations

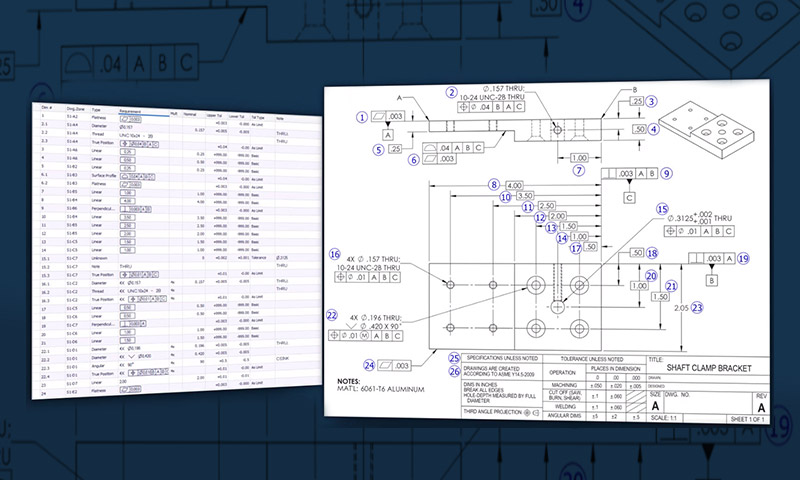
Control Plans
Products at GCM undergo a detailed control plan where each feature of your product is identified and aligned with the proper method and frequency of inspection.
Metrology
GCM has invested in the latest in sophisticated metrology equipment.
- 14x Zeiss Contura G2 CMM technology across all GCM sites. Up to 60”x 80” capability.
- Optical comparators
- Vision systems capable to +/- 0.1um
- Laser imaging